Why Were Hospital Labs Excluded From The Initial PAMA Survey?
Why Were Hospital Labs Excluded From The Initial PAMA Survey?
A former CMS official involved in drafting the initial Medicare rules that determined which labs must report their private-payer pricing data to CMS for calculating Medicare CLFS rates says that PAMA was specifically designed to exclude hospital labs. This flies in the face of the lab industry’s contention that the PAMA law intended pricing information from all labs, including hospital labs, to be included in the rate calculations.
Speaking at the Annual Meeting for the American Clinical Laboratory Assn. (ACLA), March 4, Marc Hartstein, a Principal with Health Policy Alternatives and former Director of CMS’s Hospital and Ambulatory Policy Group, said, “I provided technical assistance to the Senate Finance Committee, which wrote the statute, and I can tell you the intent was to exclude hospital laboratories. The provision was intended to get savings, and if hospital laboratories were included, that would have raised the payment amounts.”
Hartstein spent 26 years at CMS (1990-2016) and helped develop such major Medicare policies as the misvalued code initiative for the physician fee schedule, the hospital Diagnosis-Related Group system, the hospital two-midnight rule, as well as the regulations for implementing Medicare’s
new CLFS under PAMA.
At this point, the opinions of those involved in drafting the PAMA statute don’t really matter, said Hartstein, who noted that it’s now up to the court to issue a statutory interpretation of the law. “Courts rightly decide issues based on the words of the law, not the opinions of those involved in drafting or enacting the law,” he said. ACLA’s lawsuit challenging the implementation of PAMA (originally filed in December 2017) is now awaiting a ruling from Judge Amy Berman Jackson from the U.S. District Court for the District of Columbia. Judge Jackson initially dismissed the case, but ACLA won an appeal, and the case was sent back to her to make a ruling. All briefs and replies were submitted to Judge Jackson at the end of January, and a decision is expected by year’s end.
“The question is whether the secretary’s definition of ‘laboratory’ is a reasonable definition,” said Hartstein. “If a laboratory is only a laboratory and not its larger organization, the laboratory is going to get 100% of its [Medicare] revenues from the clinical laboratory fee schedule or physician fee schedule. I don’t understand what the majority revenues criterion would be in that circumstance. The majority of revenue criterion must have been drafted to eliminate somebody from this determination.”
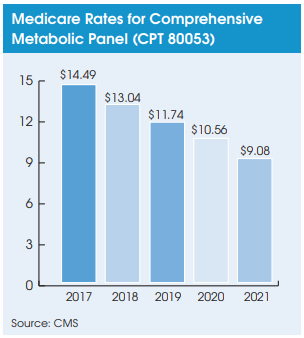
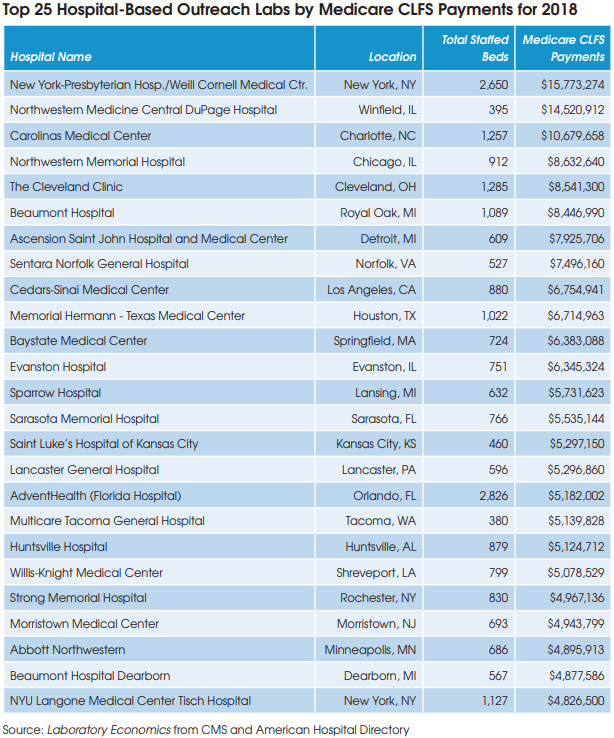
Regardless, the second PAMA reporting cycle now requires hospital labs to report their privatepayer data for non-patients to CMS in the first quarter of 2021. The hospital data, along with data from independent labs and POLs, will be used to calculate Medicare CLFS rates for 2022-2024.
Finally, the Medical Payment Advisory Commission (MedPAC) is currently reviewing how CMS has implemented the private-payer-based CLFS under PAMA, giving the lab industry an opportunity to make its case for a different system, said Hartstein. The lab industry wants CMS to analyze the payment data it collects from labs using statistical sampling to ensure that all sectors
of the lab market are accurately represented.