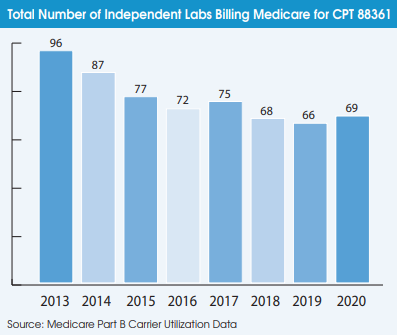
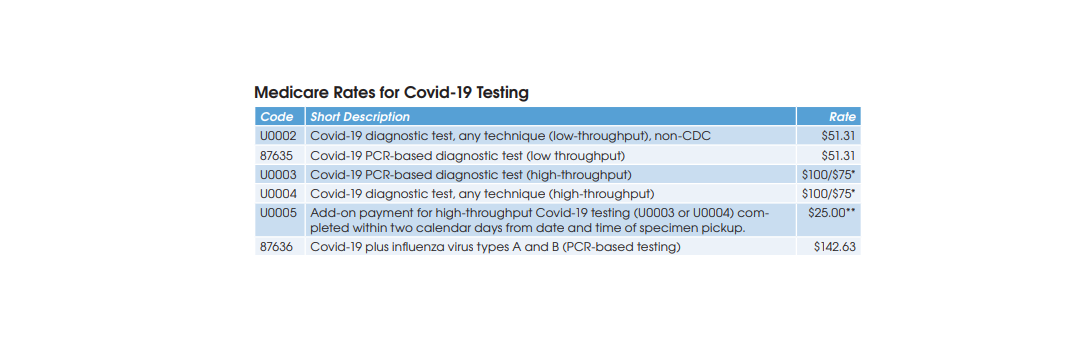
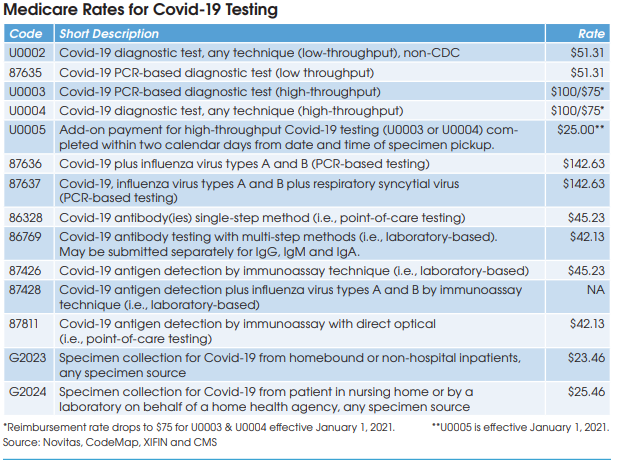
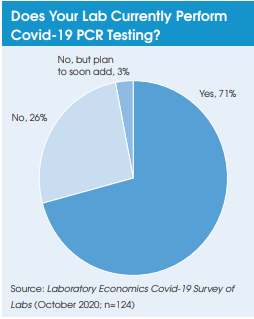
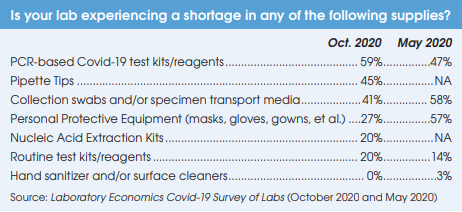
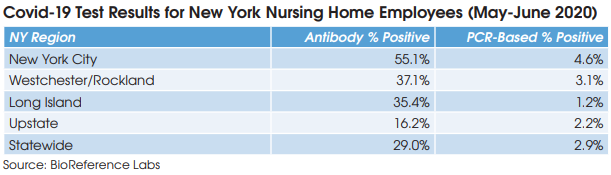
Preliminary results from a growing number of antibody prevalence studies indicate that the Covid-19 virus has spread more widely and has a lower fatality rate than previously expected.
Stanford University School of Medicine
Between 48,000 and 81,000 residents in northern California had been infected by Covid-19 as of April 1. This is more than 50 times higher than the official count at the time of 956 cases, according to a prevalence study conducted by researchers at the Stanford University School of Medicine. The Stanford study was based on 3,300 blood samples that were taken from volunteers in Santa Clara County in early April and tested for antibodies to Covid-19. Based on 100 estimated Covid-19 deaths in the county and 48,000-81,000 cases, the Stanford researchers estimate an infection fatality rate of between 0.1% and 0.2%. The Stanford research team is conducting similar antibody prevalence studies in Los Angeles County as well as a national study of 10,000 athletes and employees from 27 Major League Baseball teams. “We’re hoping that once we get accurate numbers in place, we’ll be able to quell the fear that’s out there,” said Jay Bhattacharya, who holds an MD and PhD in economics from Stanford University.
Massachusetts General Hospital
Pathologists with Massachusetts General Hospital have found that Covid-19 is far more widespread than the official case count in the Boston area. MGH set up a testing tent in the middle of Bellingham Square in Chelsea, MA, in mid-April and took finger-prick blood samples from 200 healthy-looking residents. Samples were tested using a ten-minute rapid test made by BioMedomics. The device hasn’t yet been approved by the FDA, but MGH has validated the test. The researchers found that one third of study participants (64 people) had Covid-19 antibodies. “The bad news is that there’s a raging epidemic in Chelsea, and many people walking on the street don’t know that they’re carrying the virus, according to John Iafrate, MD, PhD, Vice Chairman of MGH’s pathology department and the study’s principal investigator. “On the good-news side, it suggests that Chelsea has made its way through a good part of the epidemic.”
University of Bonn
Preliminary results from a study focused on a small German town named Gangelt indicate that about 15% of its population has been infected Covid-19. Located near the border with the Netherlands, Gangelt has been dubbed “Germany’s Wuhan” because it was hard hit by Covid-19 after a February 15 carnival celebration drew thousands to the town (population: 12,529). In early April, researchers from the University of Bonn performed antibody testing on 1,000 people from the town and found that 15% of the population had been infected and the process towards herd immunity is already taking place. The mortality rate among the studied population was 0.37%, five times lower than that currently registered in Germany, which corroborates the suspicions that the number of infected is much higher than the diagnosed. “It is important to obtain this data in order to make sure that decisions are taken based on facts rather than assumptions,” according to Hendrik Streeck, MD, PhD, Head of the Institute of Virology and Institute for HIV Research, University Hospital Bonn.