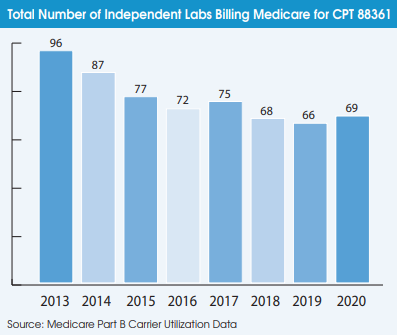
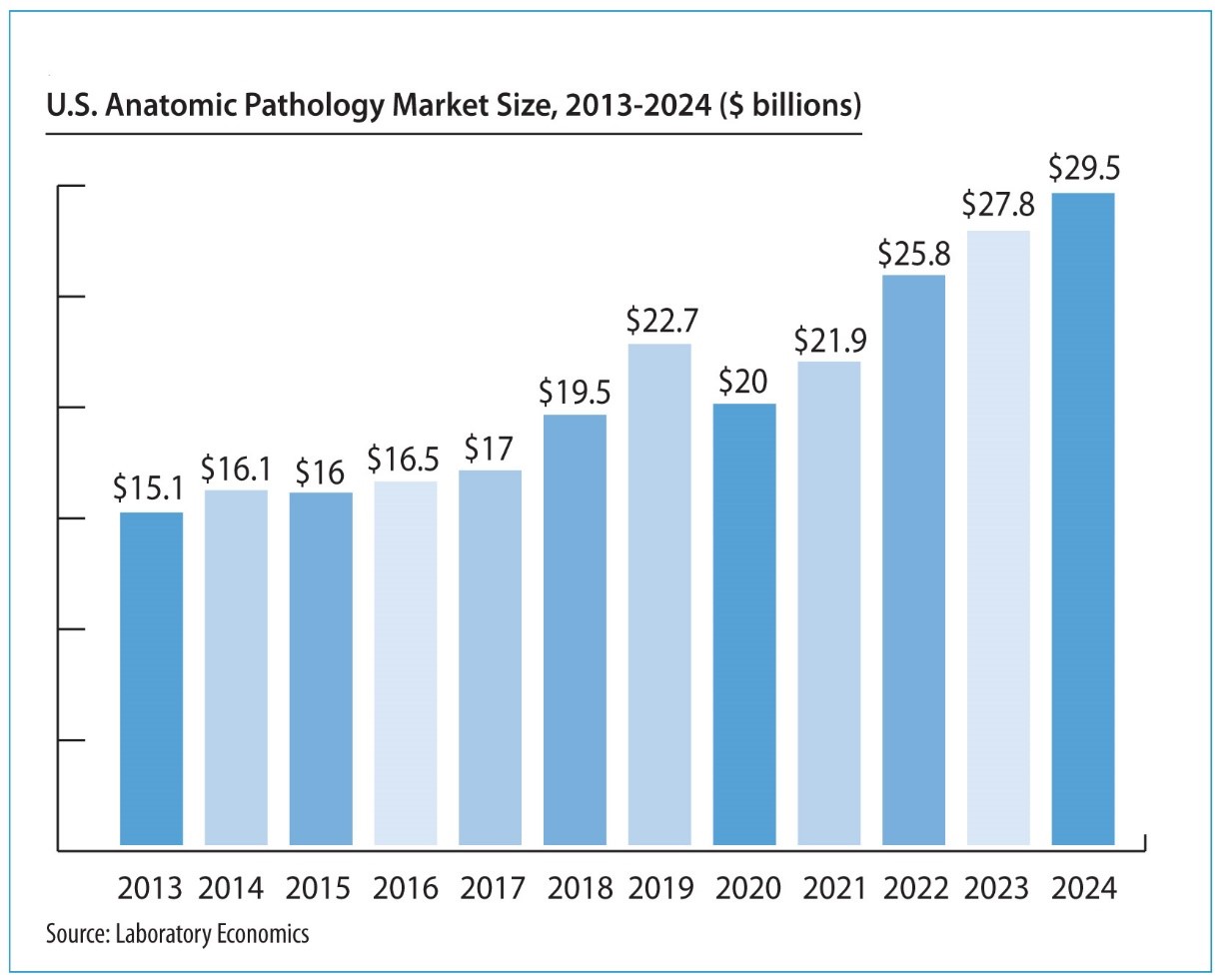
Spotlight Interview with Proscia’s Nathan Buchbinder
Spotlight Interview with Proscia’s Nathan Buchbinder
Proscia Inc. (Philadelphia, PA) markets a digital pathology software platform (Concentriq) that helps upload, organize into patient cases, annotate, and store whole slide images. Concentriq is currently being used by more than 6,000 scientists and pathologists at 300+ clinical and research organizations around the world. Proscia has also developed AI-based applications, including a program for quality control of digitized images (currently for the research market only). Here’s a summary of our recent interview with Nathan Buchbinder, Co-Founder & Chief Product Officer at Proscia.
Describe when and who founded Proscia.
Proscia was founded in 2014 by myself and two other computer scientists from Johns Hopkins University and University of Pittsburgh. These include our Chief Executive David West and our Chief Technology Officer Coleman Stavish. We currently have 100 employees.
How much capital has Proscia raised?
We raised $37 million in June 2022 bringing our total funding to $72 million. More than 10 private equity firms have invested in Proscia, including the following that have board seats: Emerald Development Managers, Flybridge Capital Partners, Razor’s Edge Ventures and Scale Venture Partners.
Why were drug development research firms so quick to adopt digital pathology?
Because their return on investment (ROI) on digitizing slides was self-evident and nearly immediate. Ten of the top 20 pharmaceutical companies, including Amgen, Bayer and Bristol Myers Squibb, are using Proscia to help manage their digitized slide images. These companies operate research sites and collaborate with third-party contract research organizations all around the world. Concentriq gives them a single hub where digitized slide images can be accessed and shared.
What’s the current status of digital pathology for clinical diagnostics?
Adoption started much slower in the clinical market. Some of our early adopters, including Thomas Jefferson University Hospitals and Johns Hopkins’ Department of Pathology, initially used digital pathology primarily for research and education.
However, over the past two years, we’ve seen a huge surge in demand from the clinical market, including integrated delivery networks, reference labs and even smaller pathology practices (~5 pathologists). These labs are using digital pathology for peer reviews, conferencing, consults, and tumor boards, as well as primary diagnosis of cancer cases. Most of our customers are in life sciences and research, but that’s quickly changing.
What’s your advice for pathology labs planning to transition to digital pathology?
Number one, get everyone involved at the start, not just executives and pathologists, but lab managers and histotechs. Number two, don’t underestimate the value of having an archive of digitized slides, not only in terms of internal research and education, but also its value to third-
party life sciences and pharmaceutical companies.
What’s your outlook for digital pathology adoption in the United States?
It will be widespread with nearly 100% adoption within five years. Drivers include the new Category III CPT codes for digital pathology and the potential for Medicare reimbursement. In addition, the application of AI, which requires digitized slides, will increase pathologist accuracy and efficiency.
The shift from microscope to monitor will be transformational. Winners and losers will be determined based on how fast and how well they implement technology. It could help the biggest commercial labs gain share in anatomic pathology or result in a different outcome that we can’t imagine today.