CAP Lobbies for 6.6% Medicare Rate Hike
CAP Lobbies for 6.6% Medicare Rate Hike
The College of American Pathologists (CAP), as well as the American
Medical Association (AMA), are lobbying Congress to pass a bill that
would hike Medicare payments to pathologists and other physicians by
6.6%. The potential rate hike would become effective April 1, and would
more than offset the 2.8% rate cut that took effect January 1, 2025.
CAP Lo Co o 6.6% M R Hk (cont’d from page 1) The bipartisan bill is called the Medicare Patient Access and Practice Stabilization Act of 2025 (H.R. 879). This bill would raise the conversion factor (CF) used to set Medicare rates for all physicians by 6.6% to $34.49 effective April 1. It would reverse the 2.8% payment cut that took effect on January 1, while also granting a payment adjustment for inflation plus a bump to reflect the lower CF in effect during the first quarter of 2025.
With the federal government facing a March 14 funding deadline, the best opportunity for passage is for this bill to be packaged with a larger spending bill to keep the government open, CAP President Donald Karcher, MD, tells Laboratory Economics. He says that the House could certainly pass it as a standalone bill because representatives have overwhelmingly supported similar Medicare payment increases for physicians in the past. But getting it through the Senate by itself would be challenging.
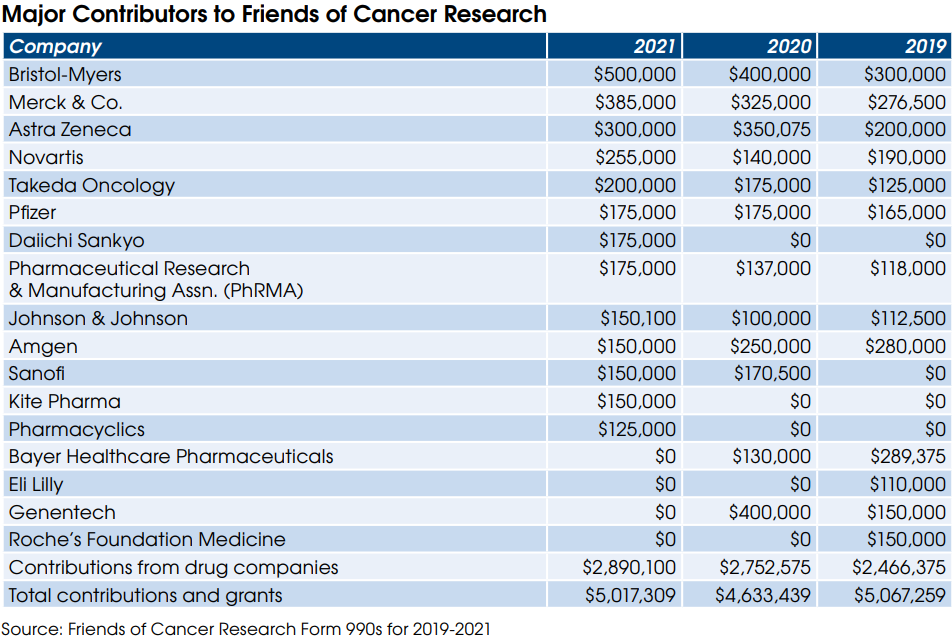
In addition to CAP and AMA, dozens of other physician groups support the bill, including the Medical Group Management Association (MGMA), the California Medical Association and the American Society for Clinical Pathology (ASCP).
The bill was introduced by Rep. Greg Murphy (R-NC) on January 31 and currently has 49 cosponsors (25 Republicans/24 Democrats).
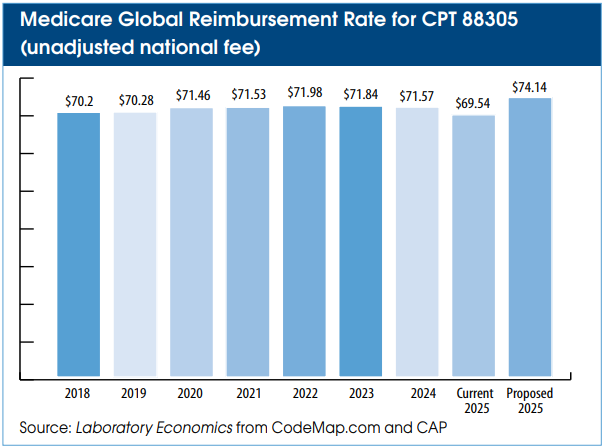
If passed into law, Medicare Physician Fee Schedule reimbursement for CPT 88305 (Level IV, tissue exam) would rise by 6.6% to a global rate of $74.14 from its current rate of $69.54. The Medicare rate hike would impact other pathology services as well as influence the rates paid by Medicaid and private health insurance plans.
Physician groups argue that a rate hike is needed to offset inflationary pressures. CMS has estimated that the Medicare Economic Index (MEI), a cumulative measure of the individual costs of running a practice, will increase by 3.5% this year after a 4.6% increase in 2024.